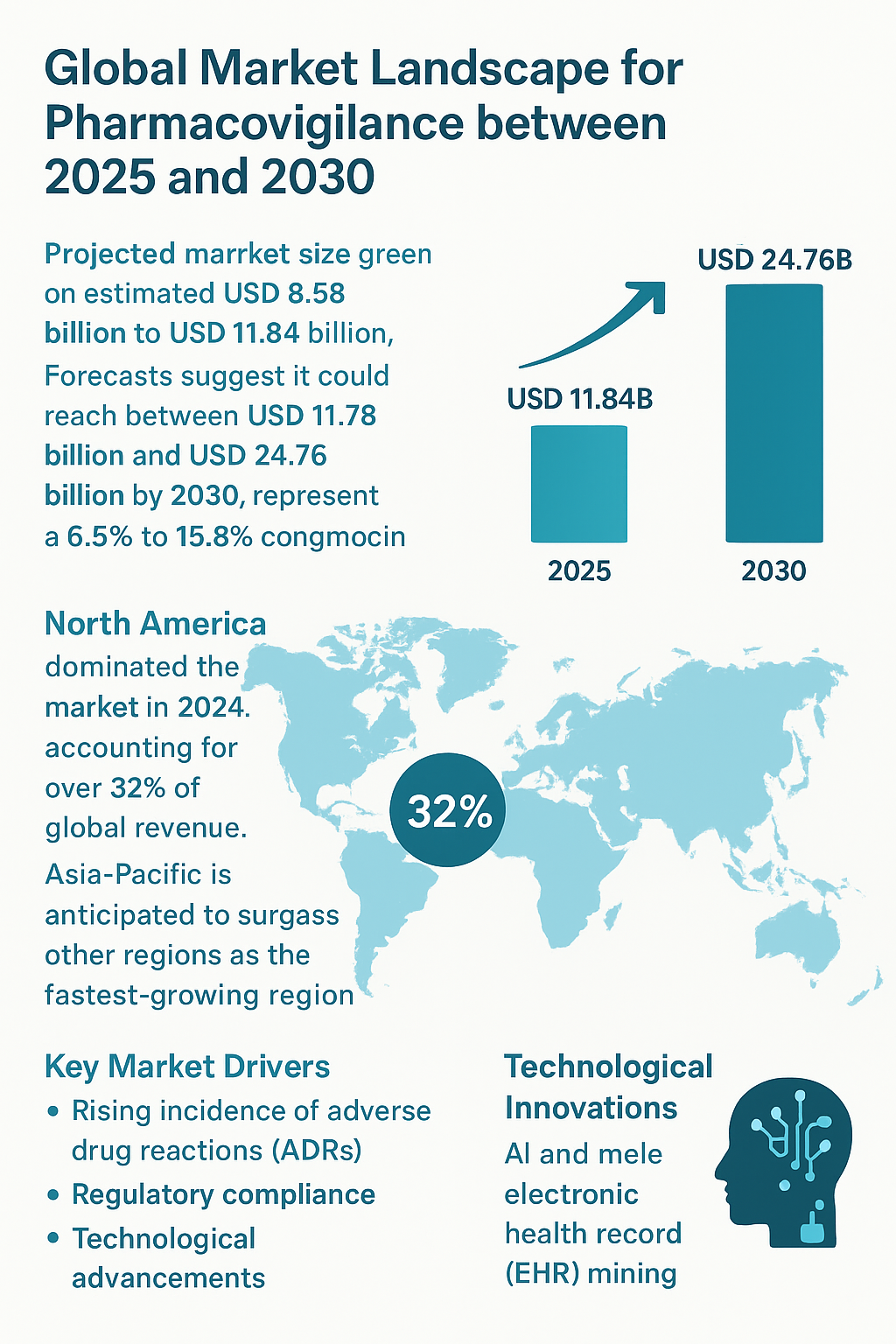
The global pharmacovigilance market is set to witness substantial growth between 2025 and 2030, driven by rising drug consumption, evolving regulatory frameworks, and technological innovations in drug safety monitoring. In 2025, the market is estimated to range between USD 8.58 billion and USD 11.84 billion, with forecasts suggesting it could reach anywhere from USD 11.78 billion to USD 24.76 billion by 2030. This represents a compound annual growth rate (CAGR) ranging from 6.5% to 15.8%, highlighting the sector’s robust expansion potential.
North America currently dominates the market, contributing over 32% of global revenue, largely due to high pharmaceutical consumption and stringent regulatory requirements. Meanwhile, Asia-Pacific is expected to emerge as the fastest-growing region, fueled by expanding healthcare infrastructure and increasing adoption of pharmacovigilance services. A major driver of this growth is the rising incidence of adverse drug reactions (ADRs), which has amplified the need for more sophisticated post-marketing safety surveillance systems. Pharmaceutical companies are increasingly investing in pharmacovigilance to ensure compliance with global regulations and maintain patient safety.
Technological advancements are reshaping the pharmacovigilance landscape. Artificial intelligence (AI) and electronic health record (EHR) mining are streamlining the detection and reporting of adverse events, while social media analytics is enabling companies to uncover previously overlooked safety signals. Contract outsourcing of pharmacovigilance services is expanding rapidly, holding over 55% of the market share in 2024 and expected to grow at a CAGR of 13.7% through 2030. Spontaneous reporting remains a significant method for adverse event collection, accounting for 43.45% of the market, but AI-driven EHR mining is projected to grow at 14.36% CAGR, demonstrating the shift toward more automated and data-driven approaches.
The integration of AI and advanced analytics ensures faster, more accurate safety assessments, helping pharmaceutical companies respond more efficiently to potential risks. Social media monitoring also adds an innovative layer, allowing stakeholders to identify and analyze adverse reactions in real time. Overall, the pharmacovigilance market is entering a period of dynamic transformation, with a focus on enhancing patient safety, regulatory compliance, and operational efficiency. Stakeholders in the pharmaceutical and healthcare sectors are likely to benefit from embracing these innovations, positioning themselves at the forefront of a rapidly evolving global market.