AI-Driven Data Management in Clinical Trials
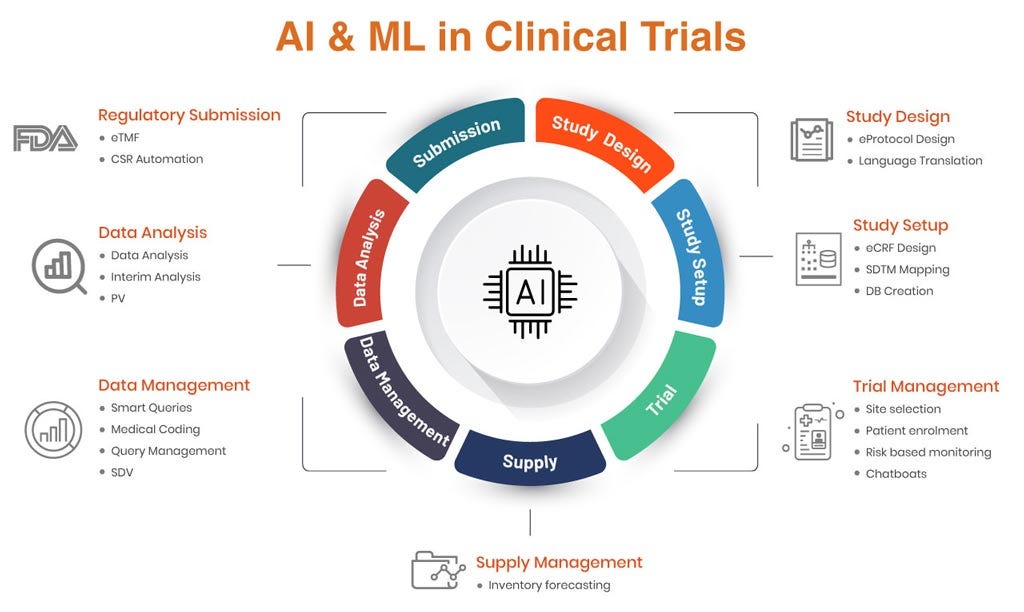
Clinical Data Management (CDM) has always been the backbone of clinical research—ensuring accuracy, compliance, and integrity of trial data. But…
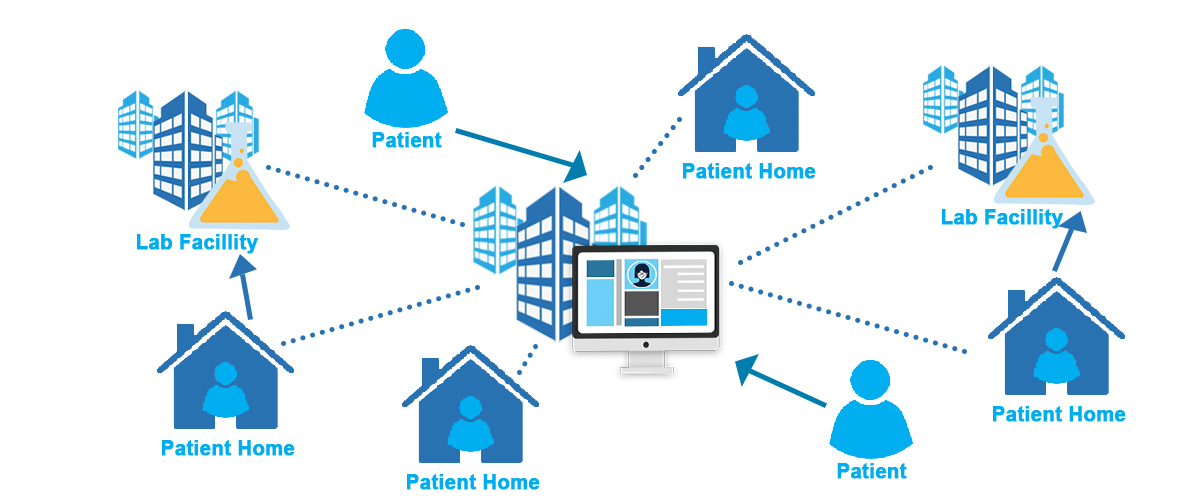
Decentralized & Hybrid Trials: Redefining Data Capture
The clinical research industry is undergoing a profound transformation. The move toward Decentralized Clinical Trials (DCTs) and hybrid models is…
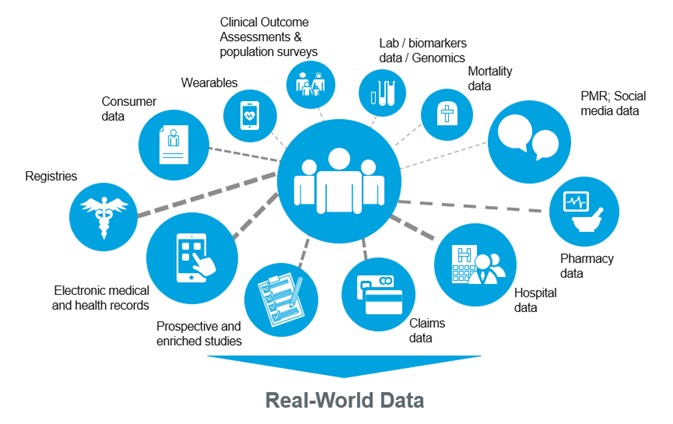
Real-World Data (RWD) & Real-World Evidence (RWE): Transforming Clinical Trials
In recent years, Real-World Data (RWD) and Real-World Evidence (RWE) have moved from being buzzwords to becoming central elements of…
Wearables & Patient-Generated Data: Managing Volume, Variety, and Value
The future of clinical research is being shaped by a single powerful trend: the rise of wearables and patient-generated health…
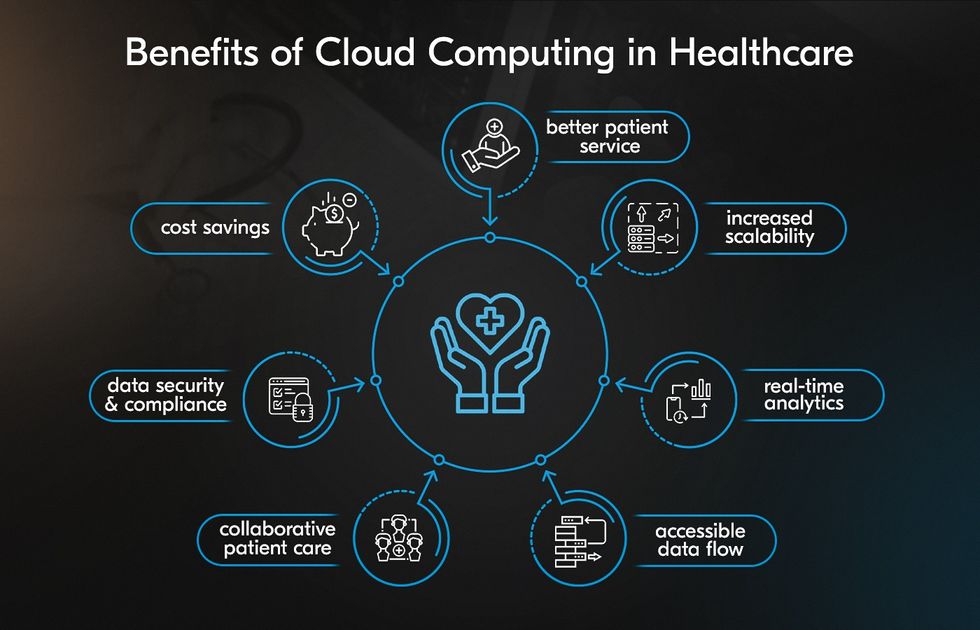
Cloud-Native & Scalable CDMS: Building the Data Foundations of Modern Trials
The landscape of clinical trials is changing rapidly. With the rise of decentralized trials, wearable technologies, and real-world data integration,…
Pfizer Associate Safety Data Management Specialist Job 2025 in Chennai – How to Apply, CV Tips, and Interview Questions
Pfizer, one of the world’s leading pharmaceutical companies, is hiring for the role of Associate Safety Data Management Specialist in…