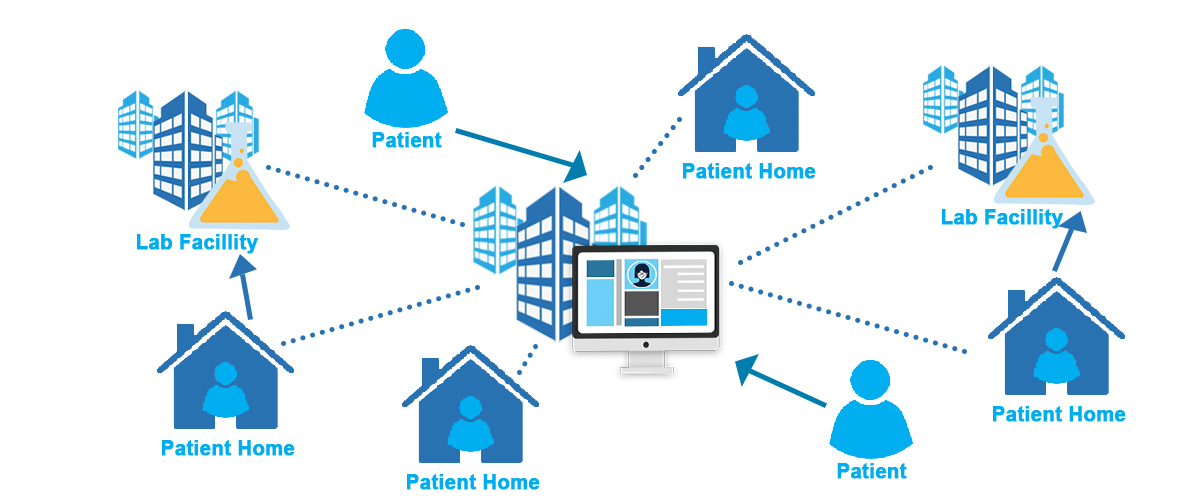
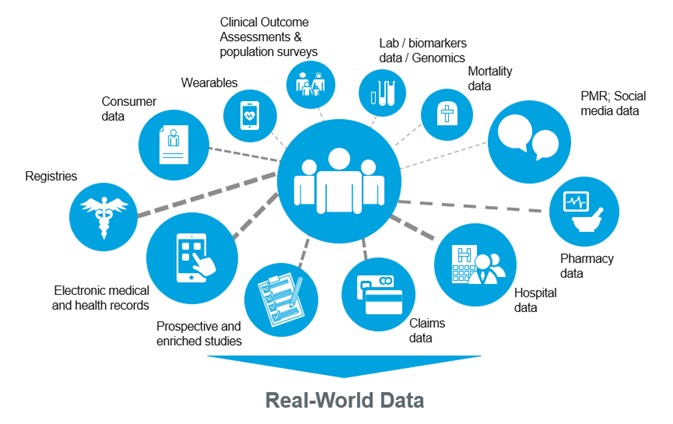
The landscape of clinical trials is changing rapidly. With the rise of decentralized trials, wearable technologies, and real-world data integration, clinical data management systems (CDMS) are under immense pressure to handle higher data volumes, greater complexity, and evolving regulatory demands. Traditional on-premise CDMS platforms are no longer sufficient to meet these challenges.
Enter cloud-native and scalable CDMS—a new generation of platforms designed to be flexible, secure, and future-ready.
Why Cloud-Native CDMS?
- Scalability:
Trials today are not limited to a handful of study sites. A global, multi-country oncology trial can generate terabytes of data from wearables, electronic health records, and ePRO (electronic patient-reported outcomes). Cloud-native CDMS platforms scale up seamlessly to handle this data load without major infrastructure overhauls. - Agility:
Hybrid and decentralized trials demand frequent protocol amendments and faster database builds. A cloud-native CDMS allows sponsors and CROs to roll out changes in weeks instead of months, reducing delays in patient recruitment or trial continuation. - Real-Time Insights:
With cloud integration, data can be captured, cleaned, and analyzed in near real-time. This empowers sponsors to make quicker decisions—such as pausing an unsafe arm of a trial or expanding a promising cohort.
Real-World Tools and Adoption
Several leading CDMS providers have already embraced the cloud-first approach:
- Medidata Rave EDC (Dassault Systèmes): Widely used in pharma, it supports complex global trials with scalability and integration into the broader Medidata Clinical Cloud ecosystem.
- Oracle Clinical One: Offers a unified platform covering randomization, trial supply management, and data capture, built for flexibility and scalability.
- Veeva Vault CDMS: Designed as a cloud-native solution from the ground up, it enables faster database builds and seamless integration with Veeva’s eTMF and CTMS products.
- Clario & Labcorp’s Covance systems: Both leveraging modular, cloud-enabled data management to streamline global studies.
For example, a large biopharma conducting a rare disease trial across North America and Europe used Veeva Vault CDMS to cut database build times by nearly 50%. This accelerated first-patient-in timelines, allowing the company to meet critical milestones for regulatory submission.
Case Scenario: Oncology Trials with Wearables
Consider an oncology trial collecting data from wearable devices such as Fitbit or Apple Watch. These devices generate continuous streams of heart rate, activity levels, and sleep patterns. A cloud-native CDMS can:
- Ingest data directly from device APIs.
- Clean and validate automatically using AI-driven modules.
- Visualize trends in real time for clinicians to monitor patient safety remotely.
This capability not only improves patient safety but also boosts retention, as participants can stay at home rather than visit trial sites for routine checkups.
Future Outlook
Cloud-native CDMS is not just a trend—it is the foundation of tomorrow’s trials. As regulators push for greater data transparency and as trials expand into real-world evidence, the ability to securely and flexibly manage massive, diverse datasets will be a competitive advantage.
Sponsors and CROs that adopt scalable, cloud-based platforms will be better positioned to:
- Accelerate trial timelines.
- Improve patient experiences.
- Meet evolving regulatory standards with confidence.
The shift is clear: the future of clinical data management lies in the cloud.
AI-Driven Data Management in Clinical Trials
Clinical Data Management (CDM) has always been the backbone of clinical research—ensuring accuracy, compliance, and integrity of trial data. But…
Decentralized & Hybrid Trials: Redefining Data Capture
The clinical research industry is undergoing a profound transformation. The move toward Decentralized Clinical Trials (DCTs) and hybrid models is…
Real-World Data (RWD) & Real-World Evidence (RWE): Transforming Clinical Trials
In recent years, Real-World Data (RWD) and Real-World Evidence (RWE) have moved from being buzzwords to becoming central elements of…
Wearables & Patient-Generated Data: Managing Volume, Variety, and Value
The future of clinical research is being shaped by a single powerful trend: the rise of wearables and patient-generated health…
Cloud-Native & Scalable CDMS: Building the Data Foundations of Modern
The landscape of clinical trials is changing rapidly. With the rise of decentralized trials, wearable technologies, and real-world data integration,…
Pfizer Associate Safety Data Management Specialist Job 2025 in Chennai
Pfizer, one of the world’s leading pharmaceutical companies, is hiring for the role of Associate Safety Data Management Specialist in…