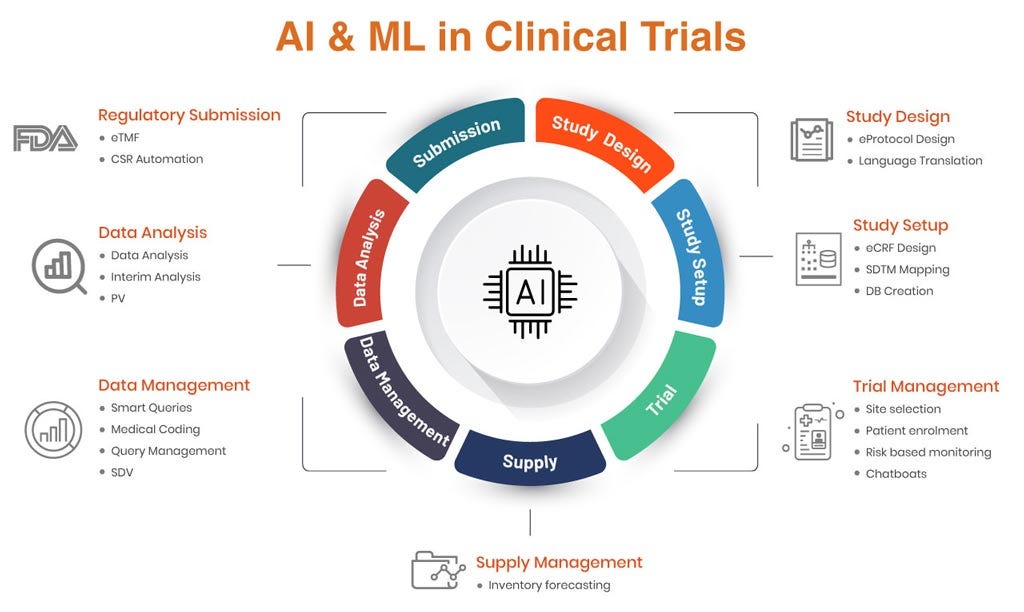
Clinical Data Management (CDM) has always been the backbone of clinical research—ensuring accuracy, compliance, and integrity of trial data. But as trials grow in complexity and scale, traditional approaches are being stretched to their limits. This is where Artificial Intelligence (AI) and Machine Learning (ML) are beginning to reshape the landscape, offering smarter, faster, and more reliable ways of managing clinical trial data.
Streamlining Data Cleaning and Query Management
Data cleaning has historically been one of the most time-consuming activities in clinical trials. AI and ML models now automate many of these processes:
- Automated anomaly detection identifies inconsistencies or outliers in patient data in real-time.
- Natural language processing (NLP) helps review unstructured data sources such as patient notes and adverse event narratives.
- Smart query management reduces the number of manual queries by predicting and preventing common data errors before they occur.
By minimizing manual intervention, AI frees up CDM professionals to focus on strategic decision-making rather than repetitive checks.
Predictive Analytics for Smarter Trials
Beyond operational efficiency, AI also adds a predictive layer to clinical research:
- Patient recruitment and retention can be optimized by analyzing historical enrollment patterns.
- Risk-based monitoring leverages predictive algorithms to identify sites or patients at higher risk of non-compliance.
- Forecasting trial timelines allows sponsors to anticipate delays and take corrective action earlier.
These applications help reduce trial duration while improving both data quality and patient safety.
Integrating AI into EDC and CTMS Platforms
The real impact of AI comes from its seamless integration with Electronic Data Capture (EDC) and Clinical Trial Management Systems (CTMS). Best practices for implementation include:
- Modular adoption: Start with AI add-ons (e.g., anomaly detection) before scaling to end-to-end automation.
- Cloud-based solutions: Ensure flexibility and scalability for handling high-volume, multi-format data.
- Interoperability standards: Use CDISC and FHIR frameworks for consistent data flow across systems.
- Change management: Train teams to work alongside AI, focusing on trust, oversight, and validation of outputs.
Adoption should not be seen as replacing human expertise but as augmenting it with faster, data-driven insights.
Case Examples of Impact
Several organizations are already reporting measurable benefits:
- Data cleaning time reduced by 30–40% when anomaly detection models are embedded in EDC systems.
- Improved data accuracy and completeness, as ML tools identify missing or inconsistent fields faster than manual review.
- Shortened trial timelines, with AI-powered predictive analytics helping sponsors anticipate recruitment challenges and proactively address them.
These early successes point toward a future where AI-driven CDM will be standard practice, not an experimental add-on.
Looking Ahead
AI and ML are not merely tools to increase efficiency—they represent a paradigm shift in clinical trial operations. For CDM professionals, the challenge lies in balancing automation with oversight, ensuring that algorithms support rather than replace human judgment.
As regulatory frameworks evolve to keep pace, AI will likely play a central role in achieving higher data integrity, faster submissions, and ultimately, quicker delivery of life-saving therapies to patients.
AI-Driven Data Management in Clinical Trials
Clinical Data Management (CDM) has always been the backbone of clinical research—ensuring accuracy, compliance, and integrity of trial data. But…
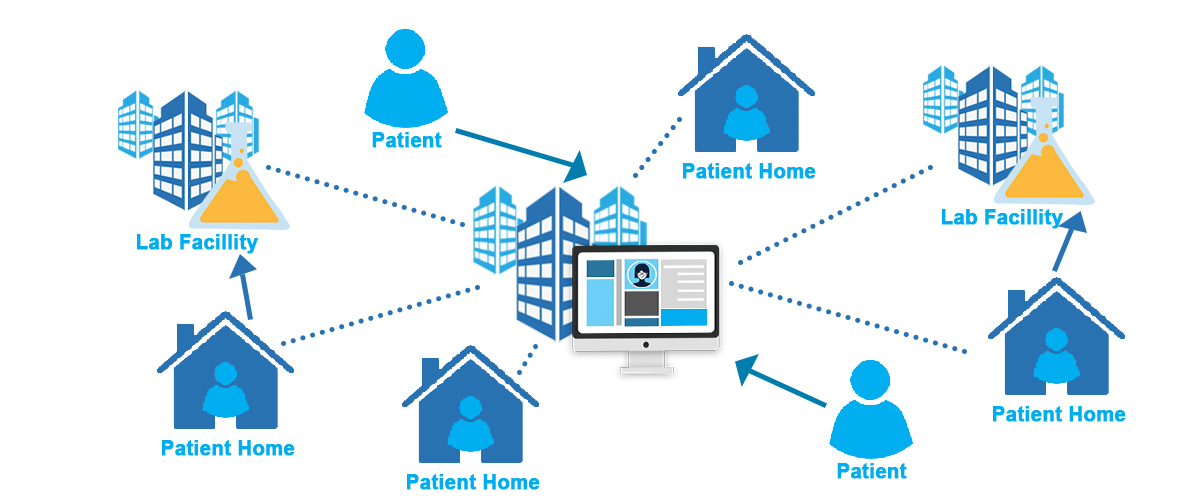
Decentralized & Hybrid Trials: Redefining Data Capture
The clinical research industry is undergoing a profound transformation. The move toward Decentralized Clinical Trials (DCTs) and hybrid models is…
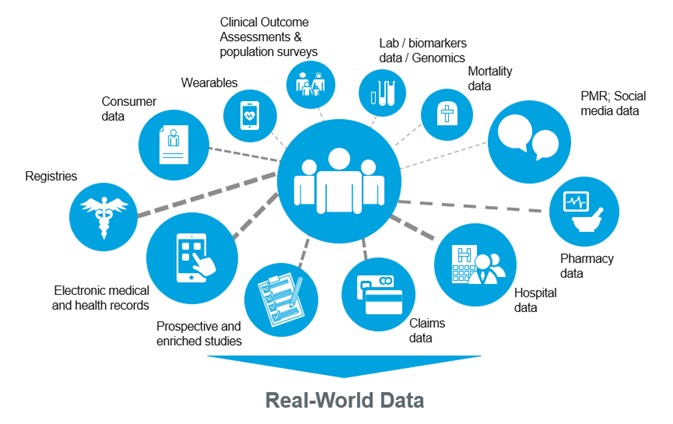
Real-World Data (RWD) & Real-World Evidence (RWE): Transforming Clinical Trials
In recent years, Real-World Data (RWD) and Real-World Evidence (RWE) have moved from being buzzwords to becoming central elements of…
Wearables & Patient-Generated Data: Managing Volume, Variety, and Value
The future of clinical research is being shaped by a single powerful trend: the rise of wearables and patient-generated health…
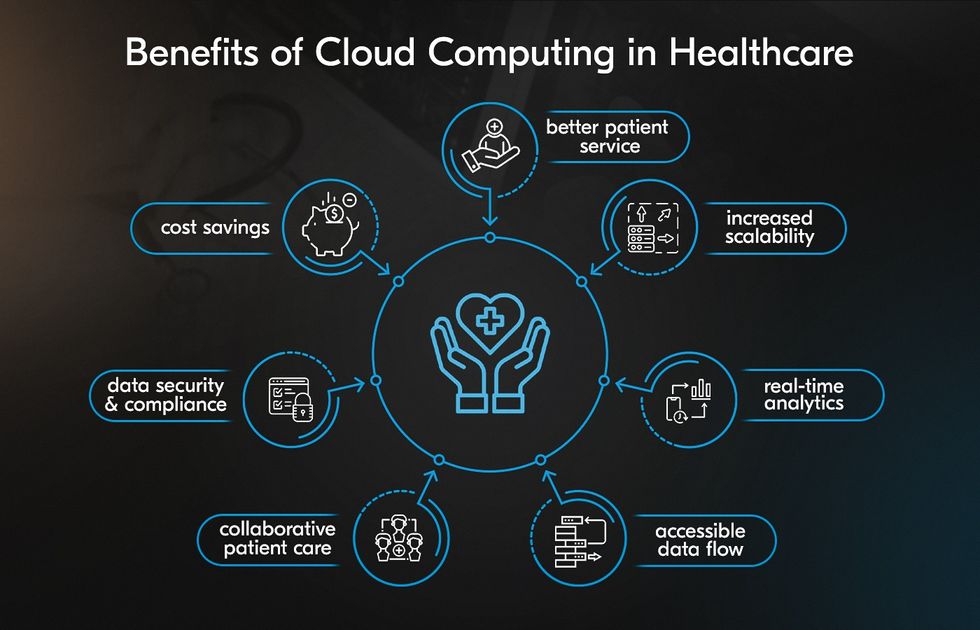
Cloud-Native & Scalable CDMS: Building the Data Foundations of Modern
The landscape of clinical trials is changing rapidly. With the rise of decentralized trials, wearable technologies, and real-world data integration,…
Pfizer Associate Safety Data Management Specialist Job 2025 in Chennai
Pfizer, one of the world’s leading pharmaceutical companies, is hiring for the role of Associate Safety Data Management Specialist in…