The clinical research industry is undergoing a profound transformation. The move toward Decentralized Clinical Trials (DCTs) and hybrid models is reshaping how data is collected, managed, and utilized. Driven by advancements in technology and patient demand for more flexible participation, these models are no longer optional—they are becoming integral to the future of clinical trials.
The Rise of Remote Monitoring and Digital Health Tools
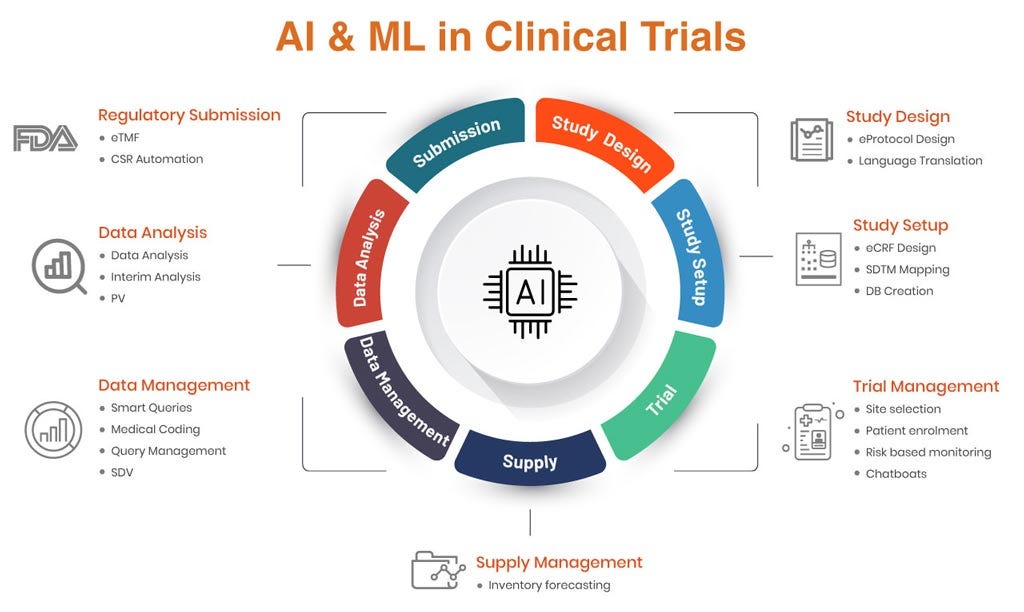
AI-Driven Data Management in Clinical Trials
Clinical Data Management (CDM) has always been the backbone of clinical research—ensuring accuracy, compliance, and integrity of trial data. But…
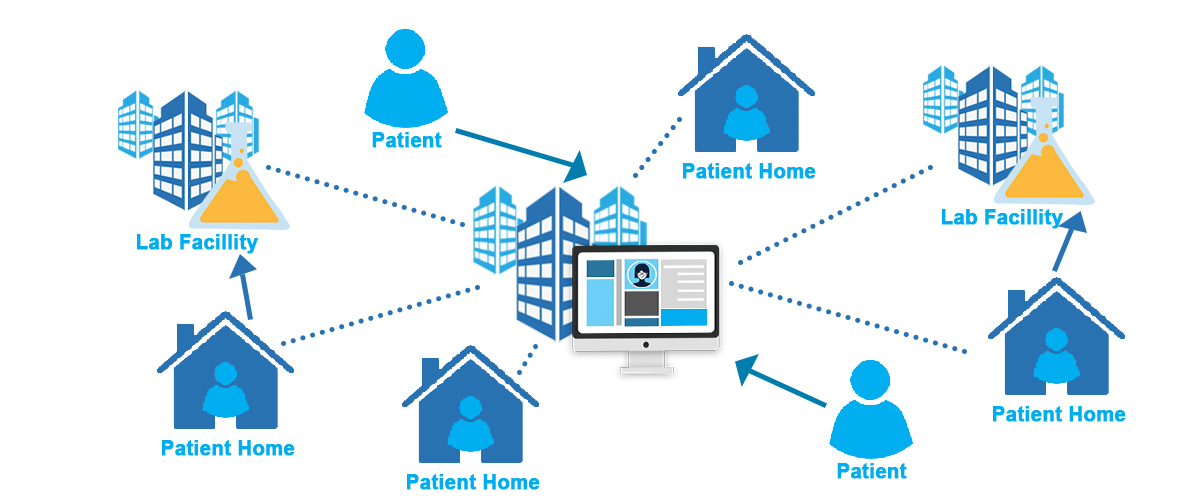
Decentralized & Hybrid Trials: Redefining Data Capture
The clinical research industry is undergoing a profound transformation. The move toward Decentralized Clinical Trials (DCTs) and hybrid models is…
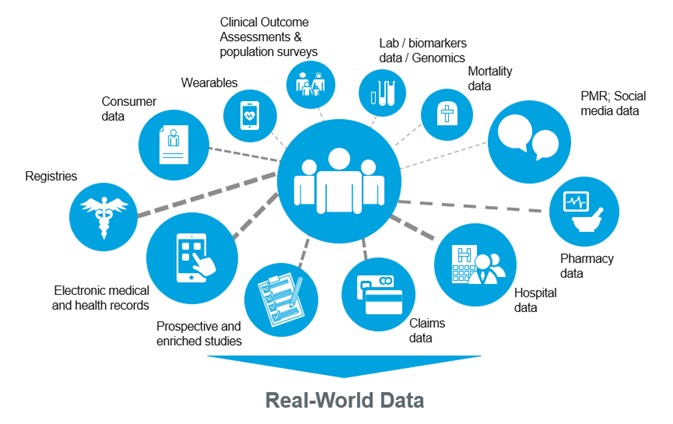
Real-World Data (RWD) & Real-World Evidence (RWE): Transforming Clinical Trials
In recent years, Real-World Data (RWD) and Real-World Evidence (RWE) have moved from being buzzwords to becoming central elements of…
Wearables & Patient-Generated Data: Managing Volume, Variety, and Value
The future of clinical research is being shaped by a single powerful trend: the rise of wearables and patient-generated health…
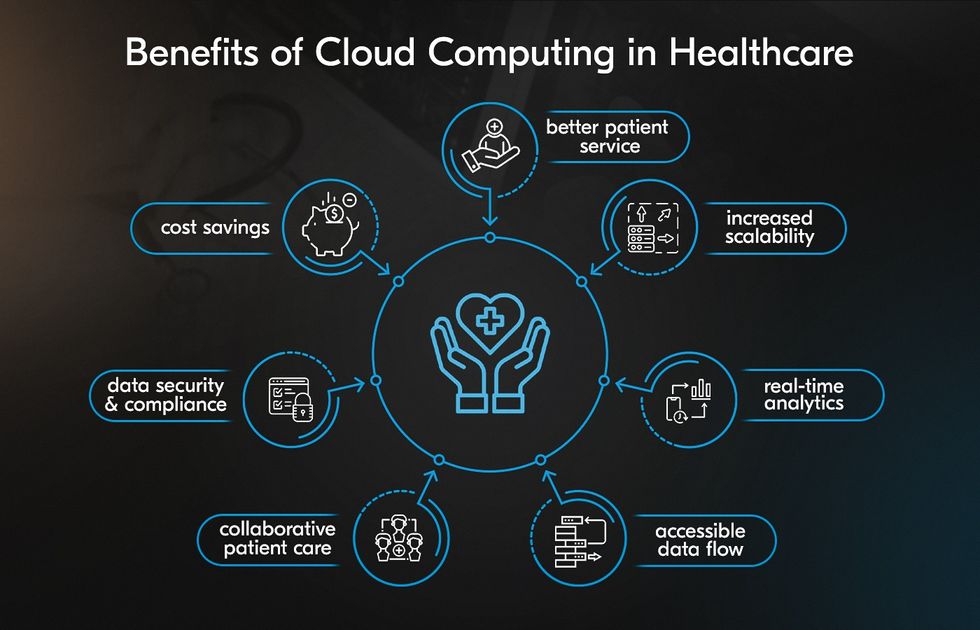
Cloud-Native & Scalable CDMS: Building the Data Foundations of Modern
The landscape of clinical trials is changing rapidly. With the rise of decentralized trials, wearable technologies, and real-world data integration,…
Pfizer Associate Safety Data Management Specialist Job 2025 in Chennai
Pfizer, one of the world’s leading pharmaceutical companies, is hiring for the role of Associate Safety Data Management Specialist in…
Decentralized and hybrid trials rely on a suite of digital technologies that extend data collection far beyond traditional clinical sites:
- Wearables and biosensors track vital signs and activity continuously, offering real-time insights into patient health.
- ePROs (electronic patient-reported outcomes) capture patient experiences directly and reduce reliance on manual reporting.
- Mobile health apps facilitate medication reminders, symptom tracking, and two-way communication between patients and researchers.
- Remote monitoring platforms enable site teams to evaluate adherence and safety without requiring frequent in-person visits.
These innovations create richer, more patient-centered datasets while improving trial accessibility for diverse populations.
Managing the Surge of Real-World Data
With these technologies comes a significant challenge: the sheer volume, variety, and velocity of data entering clinical systems. Clinical Data Management (CDM) teams are tasked with ensuring this data is accurate, reliable, and compliant.
- Volume: Continuous data streams from wearables produce massive datasets requiring scalable infrastructure.
- Variety: Data now comes in multiple formats—structured EHR data, unstructured patient reports, and real-time sensor outputs.
- Velocity: The speed of data flow demands near real-time monitoring, validation, and decision-making.
To meet these challenges, CDM teams are increasingly adopting cloud-based platforms, advanced data warehouses, and AI-driven analytics that can process and standardize information at scale.
Practical Strategies for Interoperability and Patient-Centric Design
Successfully implementing decentralized and hybrid trials requires more than just new technology. It demands a deliberate focus on interoperability and patient experience.
- Adopt Global Standards: Utilize CDISC and FHIR frameworks to ensure seamless data exchange across systems.
- Integrate Systems through APIs: Build connectivity between EDC, CTMS, and external patient-facing tools.
- Prioritize Data Privacy and Security: Incorporate encryption, anonymization, and strict governance to maintain regulatory compliance.
- Design Around the Patient: Ensure tools are intuitive, minimize participant burden, and enhance engagement.
A trial designed with patient convenience in mind not only improves recruitment and retention but also leads to higher-quality, more complete data.
Looking Ahead
Decentralized and hybrid trials are not just temporary responses to the pandemic era—they are setting the foundation for a new clinical trial paradigm. For sponsors and CROs, the key will be balancing technological innovation with regulatory compliance and patient trust. For CDM professionals, this evolution means embracing new tools, upskilling in interoperability standards, and developing strategies to manage increasingly complex data ecosystems.
The future of data capture in clinical trials will be defined by inclusivity, flexibility, and real-world relevance. Those who adapt early will be best positioned to lead in the rapidly changing research landscape.