Medical Affairs is one of the most dynamic and fast-evolving areas in the pharmaceutical industry today. For pharmacy graduates in India, this field offers significant opportunities to transition into the corporate sector. Traditionally viewed as a support function, Medical Affairs has now become a strategic driver of scientific communication, real-world evidence generation, regulatory compliance, and patient engagement.
If you are a pharmacy graduate aiming to build a career in the corporate healthcare sector, understanding the latest trends in Medical Affairs can give you a clear advantage.
Real-World Evidence and Evidence Generation
Pharmaceutical companies are increasingly looking beyond traditional clinical trial data and focusing on Real-World Evidence (RWE). Insights drawn from hospital records, electronic health databases, and patient registries are playing a major role in drug approvals and reimbursement strategies.
Pharmacy graduates who understand the basics of real-world studies, pharmacovigilance processes, and outcomes research will be more attractive to employers. Gaining knowledge of data analytics tools such as Excel, Power BI, SQL, and Python can provide an added edge.
Digital Transformation in Medical Affairs
The shift toward digital-first engagement is transforming Medical Affairs globally. Virtual advisory boards, AI-driven literature reviews, and digital interactions with healthcare professionals are now standard practices.
Graduates entering this field need to be comfortable with platforms such as Veeva, MedHub, Argus Safety, and Salesforce Health Cloud. Awareness of how artificial intelligence is applied in medical data review and scientific engagement is also becoming essential.
Patient-Centric Approach
Medical Affairs is moving from a doctor-centric model to a patient-inclusive one. Teams are now directly involved in patient advocacy, disease awareness programs, and caregiver education.
For pharmacy graduates, this means developing strong communication skills, scientific storytelling abilities, and empathy to address multiple stakeholders, including patients, physicians, and regulatory bodies.
Value-Based Healthcare and Market Access
With rising healthcare costs, pharmaceutical companies are under pressure to demonstrate the cost-effectiveness of their products. Value-based healthcare, supported by pharmacoeconomics and Health Economics and Outcomes Research (HEOR), is gaining importance.
Graduates who understand pharmacoeconomic concepts such as cost-effectiveness analysis and Quality-Adjusted Life Years (QALYs) will be well positioned for careers that connect medical affairs with market access.
The Expanding Role of Medical Science Liaisons
Medical Science Liaisons (MSLs) are now central figures in Medical Affairs. Their responsibilities extend beyond scientific discussions with healthcare professionals to include digital engagements, supporting real-world evidence studies, and leading advisory boards.
Pharmacy graduates aspiring to become MSLs must strengthen their presentation skills, clinical knowledge, and networking abilities.
Integration with Pharmacovigilance
Pharmacovigilance and Medical Affairs teams are working more closely than ever. The collaboration helps ensure timely safety reporting, detection of signals, and responses to regulatory queries.
Pharmacy graduates who are familiar with pharmacovigilance processes such as Individual Case Safety Reports (ICSRs), Periodic Safety Update Reports (PSURs), and Development Safety Update Reports (DSURs) will have a competitive edge.
Rise of Medical Communications and Scientific Content
Scientific content development is a fast-growing area within Medical Affairs. From publications and slide decks to regulatory responses and patient education materials, the demand for skilled medical communicators is on the rise.
Pharmacy graduates can stand out by improving their medical writing skills, learning to conduct literature searches on PubMed, and mastering reference management tools such as EndNote or Zotero.
Regulatory and Compliance Awareness
Global pharmaceutical companies expect Medical Affairs professionals to have a clear understanding of compliance frameworks. Knowledge of ICH-GCP, Good Pharmacovigilance Practice (GVP), GDPR, and India’s PvPI guidelines is highly valued.
For pharmacy graduates, awareness of regulatory documentation and adherence to standard operating procedures (SOPs) can significantly improve career prospects.
Cross-Functional Collaboration
Medical Affairs sits at the intersection of R&D, Pharmacovigilance, Regulatory Affairs, and Commercial teams. The ability to collaborate across departments is a key skill for future professionals.
Graduates should focus on building teamwork, project management, and business acumen to handle cross-functional responsibilities effectively.
Artificial Intelligence and Data Science in Pharma
Artificial Intelligence and Machine Learning are transforming how Medical Affairs teams operate. AI tools are being used in clinical trial monitoring, literature surveillance, KOL mapping, and safety signal detection.
Pharmacy graduates who develop familiarity with data visualization tools, basic SQL, and data-driven decision-making will be well prepared for future opportunities in this evolving space.
How Pharmacy Graduates Can Prepare for a Career in Medical Affairs
Pharmacy graduates in India who wish to enter Medical Affairs should focus on building a combination of scientific knowledge, digital skills, and communication abilities. A strong career roadmap may include:
- Gaining core knowledge in pharmacovigilance, medical writing, and basics of HEOR.
- Building technical skills with Excel, Power BI, SQL, and pharmacovigilance databases.
- Developing communication and presentation skills for stakeholder engagement.
- Pursuing certifications in pharmacovigilance, market access, or data analytics.
- Staying updated by following pharmaceutical companies such as Novartis, Pfizer, IQVIA, Optum, and Syneos Health.
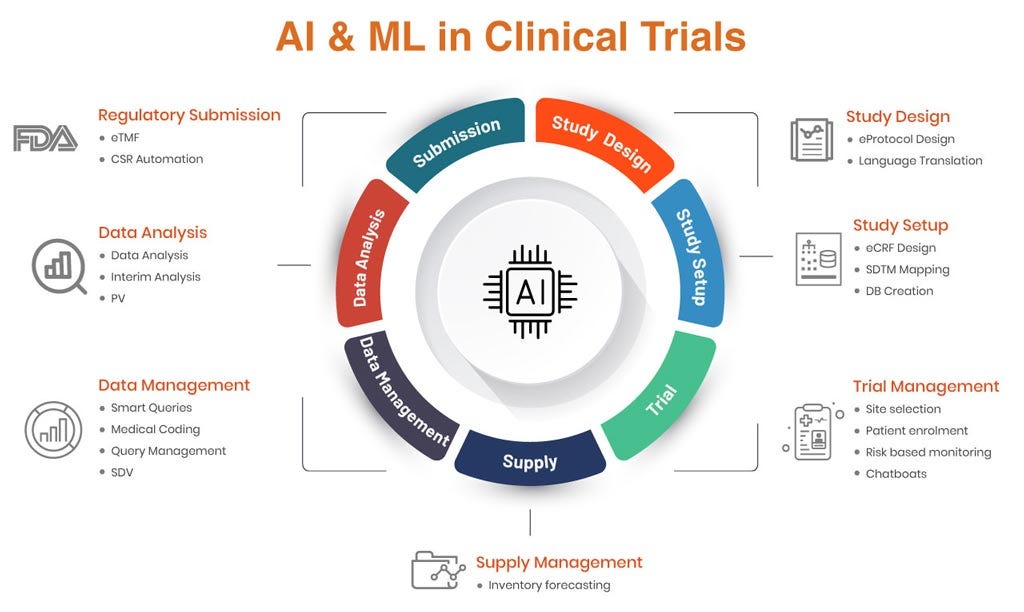
AI-Driven Data Management in Clinical Trials
Clinical Data Management (CDM) has always been the backbone of clinical research—ensuring accuracy, compliance, and integrity of trial data. But…
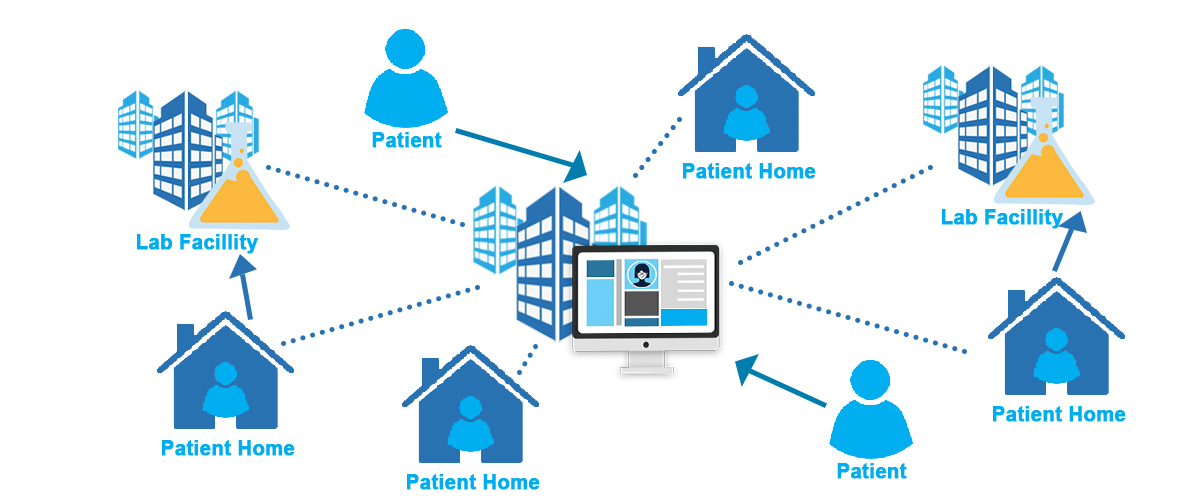
Decentralized & Hybrid Trials: Redefining Data Capture
The clinical research industry is undergoing a profound transformation. The move toward Decentralized Clinical Trials (DCTs) and hybrid models is…
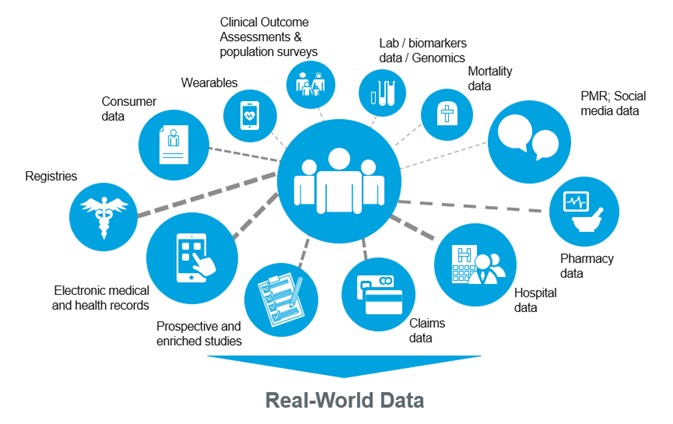
Real-World Data (RWD) & Real-World Evidence (RWE): Transforming Clinical Trials
In recent years, Real-World Data (RWD) and Real-World Evidence (RWE) have moved from being buzzwords to becoming central elements of…
Wearables & Patient-Generated Data: Managing Volume, Variety, and Value
The future of clinical research is being shaped by a single powerful trend: the rise of wearables and patient-generated health…
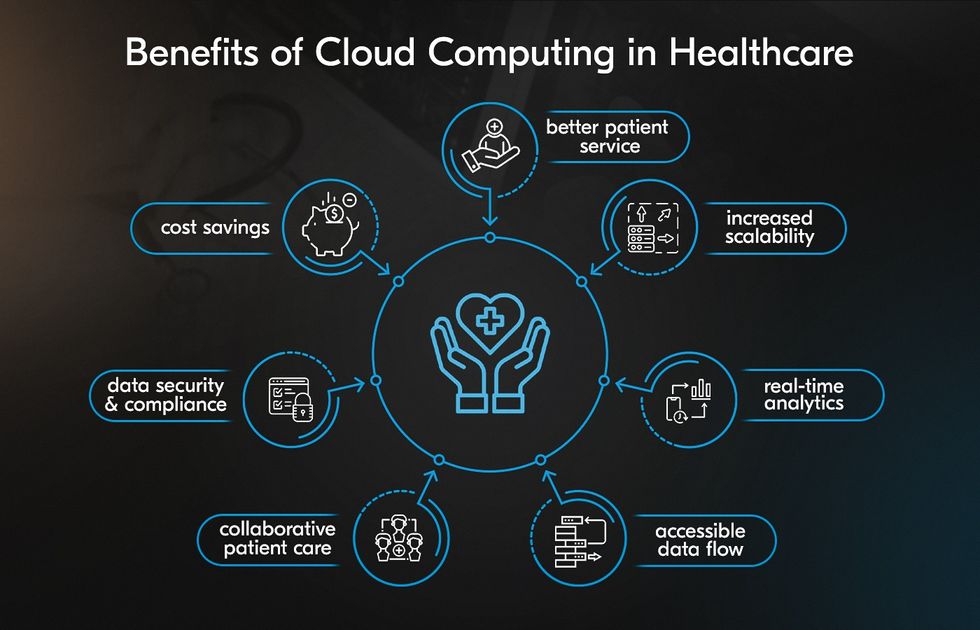
Cloud-Native & Scalable CDMS: Building the Data Foundations of Modern
The landscape of clinical trials is changing rapidly. With the rise of decentralized trials, wearable technologies, and real-world data integration,…
Pfizer Associate Safety Data Management Specialist Job 2025 in Chennai
Pfizer, one of the world’s leading pharmaceutical companies, is hiring for the role of Associate Safety Data Management Specialist in…