Pfizer, one of the world’s leading pharmaceutical companies, is hiring for the role of Associate Safety Data Management Specialist in Chennai, India. This role is a golden opportunity for B.Pharm, M.Pharm, and Pharm.D graduates who want to start their career in pharmacovigilance and drug safety. If you are a fresher or have up to two years of experience in safety data management, this could be your entry point into the global healthcare industry.
👉 Application Link: Apply here on Pfizer Careers
CV Customization Tips for Students
Most students apply with generic CVs, which often get filtered out by Applicant Tracking Systems (ATS). Here’s how you can stand out:
- Add a Strong Summary: Mention your degree, PV exposure, and interest in ICSR case processing. Example: “B.Pharm graduate with pharmacovigilance training, hands-on experience in mock ICSR processing, and knowledge of ARGUS Safety and XML (E2B R2/R3).”
- Highlight Projects/Internships: Even if academic, write them clearly. Example: “Processed 50+ mock ICSRs and drafted narratives during pharmacovigilance coursework.”
- List Technical Skills: ARGUS Safety, XML E2B, MedDRA coding, pharmacovigilance guidelines.
- Include Certifications: Any online PV or MedDRA training should be included under certifications.
- Emphasize Soft Skills: Attention to detail, communication, and teamwork are key for this role.
Common Interview Questions at Pfizer
Pfizer interviewers will test both technical knowledge and behavioral skills. Be ready for questions like:
- What is an ICSR and how is it processed in pharmacovigilance?
- Can you explain the difference between E2B R2 and R3 XML formats?
- How do you ensure accuracy while processing multiple cases under tight deadlines?
- Tell me about a time you caught an error in your work and how you fixed it.
- Why do you want to work in pharmacovigilance at Pfizer?
Prepare answers using real examples from your IASD coursework, internships, or projects.
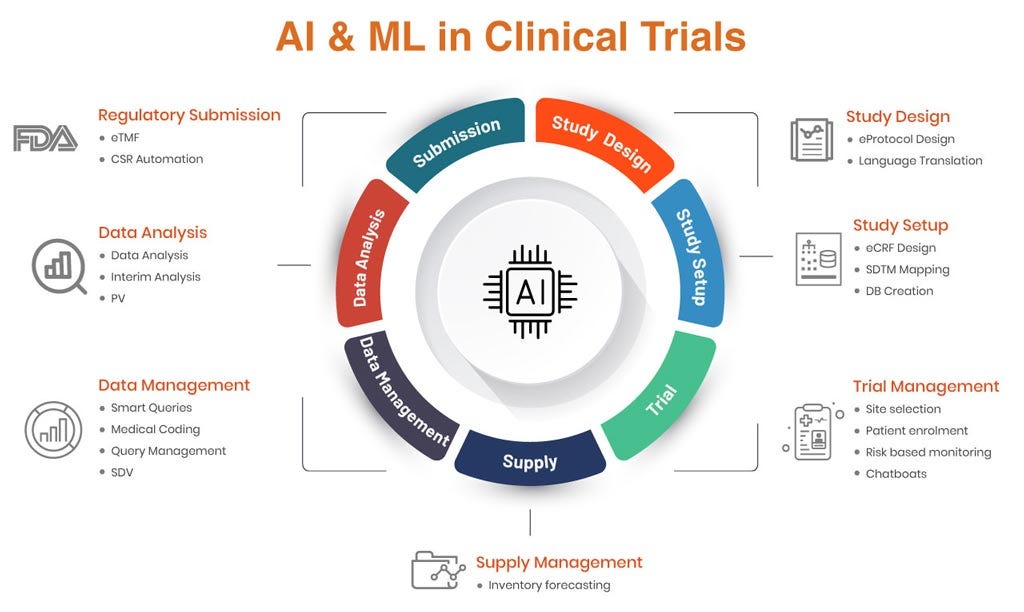
AI-Driven Data Management in Clinical Trials
Clinical Data Management (CDM) has always been the backbone of clinical research—ensuring accuracy, compliance, and integrity of trial data. But…
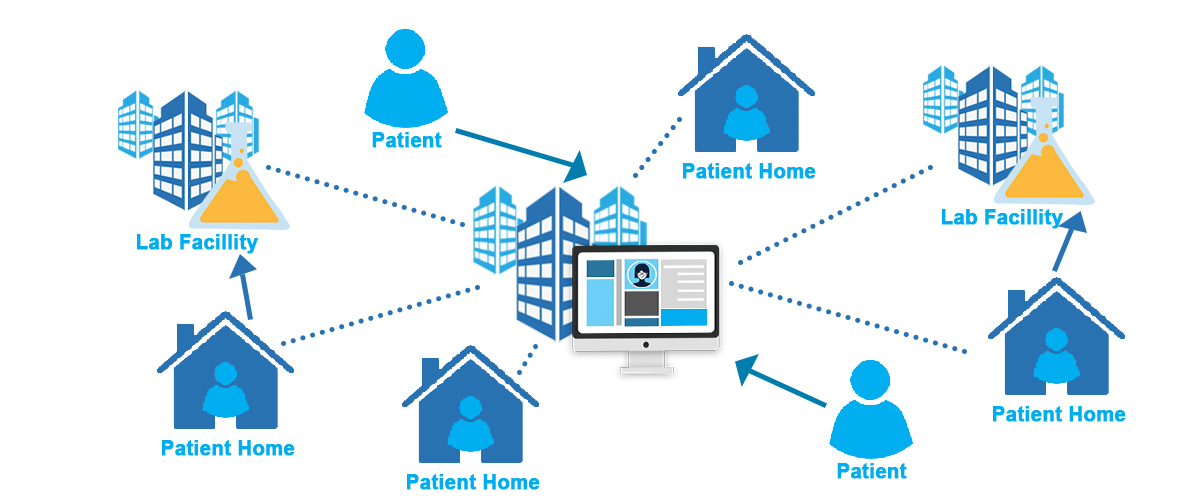
Decentralized & Hybrid Trials: Redefining Data Capture
The clinical research industry is undergoing a profound transformation. The move toward Decentralized Clinical Trials (DCTs) and hybrid models is…
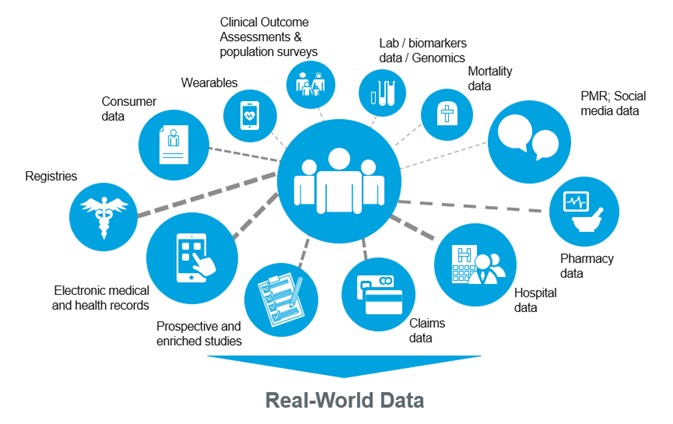
Real-World Data (RWD) & Real-World Evidence (RWE): Transforming Clinical Trials
In recent years, Real-World Data (RWD) and Real-World Evidence (RWE) have moved from being buzzwords to becoming central elements of…
Wearables & Patient-Generated Data: Managing Volume, Variety, and Value
The future of clinical research is being shaped by a single powerful trend: the rise of wearables and patient-generated health…
Cloud-Native & Scalable CDMS: Building the Data Foundations of Modern
The landscape of clinical trials is changing rapidly. With the rise of decentralized trials, wearable technologies, and real-world data integration,…
Pfizer Associate Safety Data Management Specialist Job 2025 in Chennai
Pfizer, one of the world’s leading pharmaceutical companies, is hiring for the role of Associate Safety Data Management Specialist in…



There’s a certain magic in the way you make even the most abstract ideas feel personal and real.