In recent years, Real-World Data (RWD) and Real-World Evidence (RWE) have moved from being buzzwords to becoming central elements of clinical research. Regulators such as the FDA and EMA are increasingly advocating for the use of RWD and RWE to support drug development, safety monitoring, and even regulatory decision-making. This shift signals a new era in Clinical Data Management (CDM)—one that requires advanced integration, robust data standards, and strong governance practices.
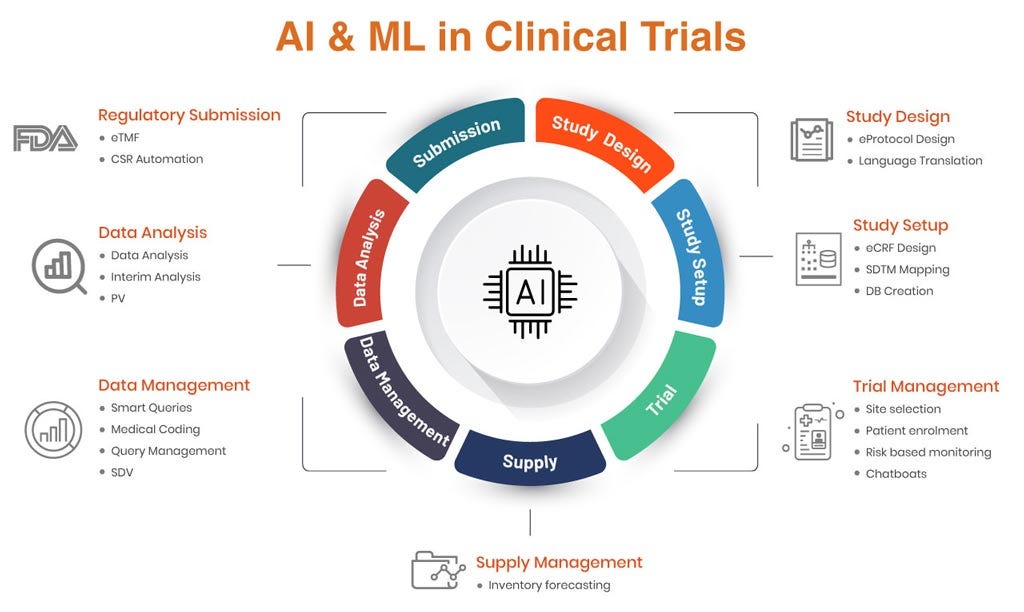
AI-Driven Data Management in Clinical Trials
Clinical Data Management (CDM) has always been the backbone of clinical research—ensuring accuracy, compliance, and integrity of trial data. But…
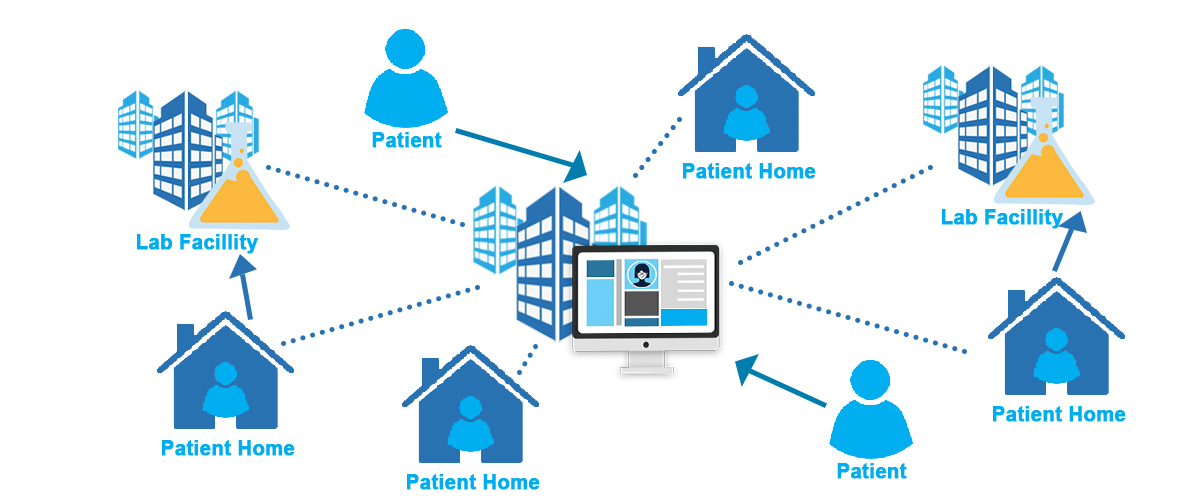
Decentralized & Hybrid Trials: Redefining Data Capture
The clinical research industry is undergoing a profound transformation. The move toward Decentralized Clinical Trials (DCTs) and hybrid models is…
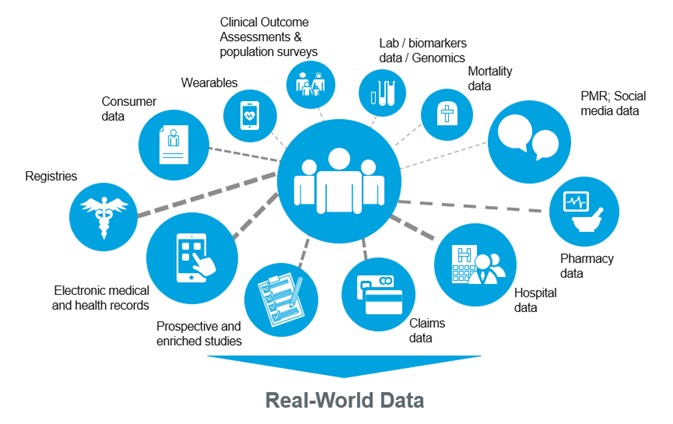
Real-World Data (RWD) & Real-World Evidence (RWE): Transforming Clinical Trials
In recent years, Real-World Data (RWD) and Real-World Evidence (RWE) have moved from being buzzwords to becoming central elements of…
Wearables & Patient-Generated Data: Managing Volume, Variety, and Value
The future of clinical research is being shaped by a single powerful trend: the rise of wearables and patient-generated health…
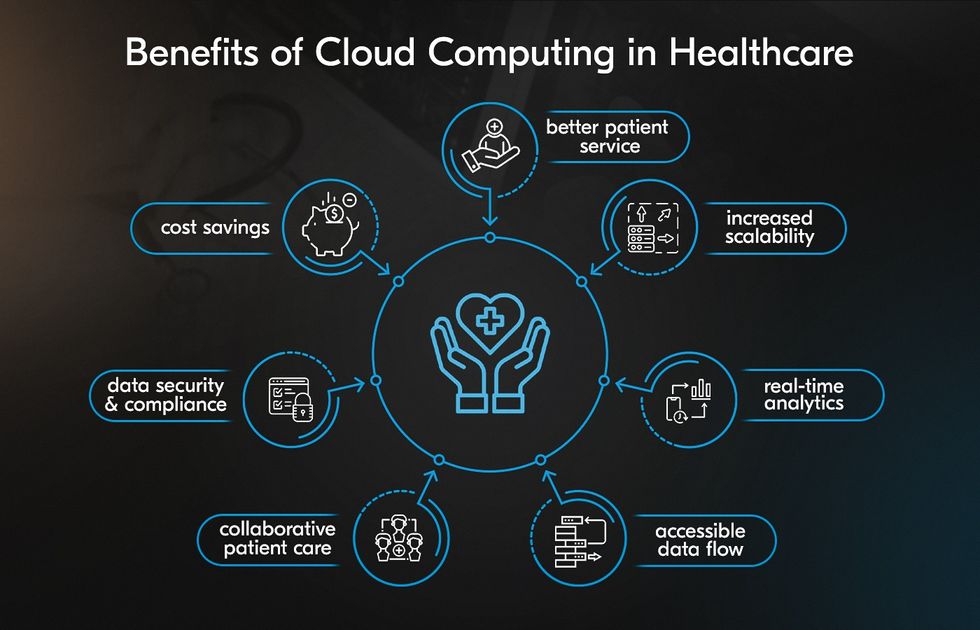
Cloud-Native & Scalable CDMS: Building the Data Foundations of Modern
The landscape of clinical trials is changing rapidly. With the rise of decentralized trials, wearable technologies, and real-world data integration,…
Pfizer Associate Safety Data Management Specialist Job 2025 in Chennai
Pfizer, one of the world’s leading pharmaceutical companies, is hiring for the role of Associate Safety Data Management Specialist in…
Why Regulators are Driving RWD Adoption
The traditional clinical trial model is rigorous but often limited in scope. Trials typically involve carefully selected patient populations under controlled conditions. While this ensures scientific validity, it can create gaps in understanding how a treatment works in diverse, real-world settings.
- FDA’s 21st Century Cures Act emphasizes the use of RWE for evaluating new indications and post-market studies.
- EMA encourages the use of RWD to monitor long-term safety and treatment effectiveness across populations.
- Regulators increasingly view RWD as a way to enhance decision-making, reduce trial costs, and improve access to innovative therapies.
Tools and Standards for Seamless Integration
Integrating RWD into clinical trials requires interoperability between systems and a commitment to globally recognized standards. Key enablers include:
- FHIR (Fast Healthcare Interoperability Resources): Supports efficient data exchange across EHRs, lab systems, and clinical trial platforms.
- CDISC Standards (SDTM, ADaM): Provide a structured approach for data submission to regulators, ensuring consistency and traceability.
- APIs and Cloud Platforms: Enable real-time integration of RWD into trial workflows, supporting faster decision-making and improved monitoring.
- Advanced Analytics Tools: Apply AI and machine learning to identify trends, detect anomalies, and generate insights from large-scale RWD datasets.
Best Practices for Ensuring Data Quality and Regulatory Acceptance
While the opportunities are immense, the inclusion of RWD and RWE demands robust governance and quality assurance. Some practical best practices include:
- Establish Clear Data Provenance: Ensure that the origin, collection methods, and transformations of data are fully documented.
- Adopt Standardized Protocols: Use CDISC and FHIR standards consistently across all data capture and integration activities.
- Implement Rigorous Data Validation: Apply automated checks and manual reviews to guarantee accuracy, completeness, and consistency.
- Prioritize Privacy and Compliance: Align data practices with HIPAA, GDPR, and emerging frameworks such as the European Health Data Space.
- Collaborate Across Stakeholders: Foster early dialogue between sponsors, CROs, technology providers, and regulators to ensure alignment.
Looking Ahead
The integration of RWD and RWE is not just a technological upgrade—it represents a fundamental transformation in how we design, execute, and evaluate clinical trials. The next few years will likely see broader adoption of decentralized and hybrid trials, where RWD plays a central role in improving diversity, patient engagement, and real-world relevance.
For Clinical Data Management professionals, this evolution brings both challenges and opportunities. Success will depend on embracing new tools, adhering to global standards, and building strong strategies for data quality and compliance.