
Pharmacovigilance (PV) has become one of the most attractive career options for B.Pharm graduates in India. With the expansion of clinical research, drug safety, and post-marketing surveillance, the demand for skilled pharmacovigilance professionals has surged. For freshers, getting a foothold in the right company can shape a strong career in drug safety and regulatory sciences.
This article lists the top pharmacovigilance companies hiring in India in 2025, along with entry-level roles, hiring trends, and salary insights.
Why Pharmacovigilance Jobs Are Growing in India
India has emerged as a global hub for pharmacovigilance outsourcing. Major pharmaceutical companies and Contract Research Organizations (CROs) are setting up drug safety centers in India because of:
- A large pool of pharmacy and life science graduates.
- Cost-effective and skilled workforce.
- Regulatory authorities mandating stronger drug safety systems.
- Increasing global clinical trials and post-marketing surveillance needs.
For B.Pharm freshers, this means abundant job opportunities in 2025.
Top Pharmacovigilance Companies Hiring in India (2025)
Below is a table of some of the leading companies hiring B.Pharm freshers in India.
| Company Name | Type | Common Fresher Roles | Typical Fresher Salary (LPA) | Locations in India |
|---|---|---|---|---|
| Accenture | IT & CRO Services | Drug Safety Associate | 3.5 – 5.5 | Bengaluru, Hyderabad, Pune |
| Tata Consultancy Services (TCS) | IT & CRO Services | PV Associate, Case Processor | 3.0 – 5.0 | Mumbai, Chennai, Kolkata, Bengaluru |
| Cognizant | IT & Healthcare | PV Associate, ICSR Processor | 3.0 – 5.0 | Hyderabad, Kolkata, Pune |
| Wipro | IT & CRO Services | Drug Safety Associate | 3.0 – 4.5 | Bengaluru, Noida, Hyderabad |
| IQVIA | Global CRO | PV Associate, Safety Data Analyst | 4.0 – 6.0 | Bengaluru, Kochi |
| Parexel | Global CRO | Safety Associate, PV Trainee | 3.5 – 5.5 | Hyderabad, Bengaluru |
| Labcorp (Covance) | Global CRO | Drug Safety Specialist (Entry) | 4.0 – 6.0 | Bengaluru |
| Novartis | Pharma Company | PV Operations Specialist | 5.0 – 7.0 | Hyderabad |
| Pfizer | Pharma Company | PV Associate | 5.0 – 7.0 | Chennai |
| Dr. Reddy’s Laboratories | Indian Pharma | PV Executive | 4.0 – 6.0 | Hyderabad |
| Sun Pharma | Indian Pharma | PV Executive | 4.0 – 6.0 | Mumbai, Vadodara |
Recruitment Trends in Pharmacovigilance Jobs
- Campus Placements: Many companies such as TCS, Accenture, and Cognizant hire B.Pharm freshers directly through campus drives.
- Walk-in Drives: Global CROs often conduct walk-in recruitment drives in Hyderabad, Bengaluru, and Pune.
- Job Portals & LinkedIn: Companies post PV openings frequently on platforms like Naukri.com, Indeed, and LinkedIn.
- Internship-to-Full-Time Pathway: Some freshers enter PV through internships and get converted to full-time roles after performance review.
Skills Required to Get Hired in Pharmacovigilance
While companies hire freshers, certain skills make you more employable:
- Knowledge of ICSR processing and MedDRA coding.
- Familiarity with pharmacovigilance software like Oracle Argus or ArisG.
- Strong understanding of drug safety regulations (ICH E2, GVP).
- Good communication skills for case narratives and reporting.
- Basic computer skills (MS Excel, Word, PowerPoint).
B.Pharm graduates who supplement their degree with short-term certification courses in pharmacovigilance are often preferred.
Salary Outlook for B.Pharm Freshers in Pharmacovigilance
Most B.Pharm freshers in pharmacovigilance start with salaries ranging from ₹3.0 LPA to ₹5.5 LPA depending on the company and city. Multinational pharma companies and CROs generally pay higher compared to IT outsourcing firms. With 2–3 years of experience, salaries can rise to ₹6–8 LPA, especially for roles in aggregate reporting and compliance.
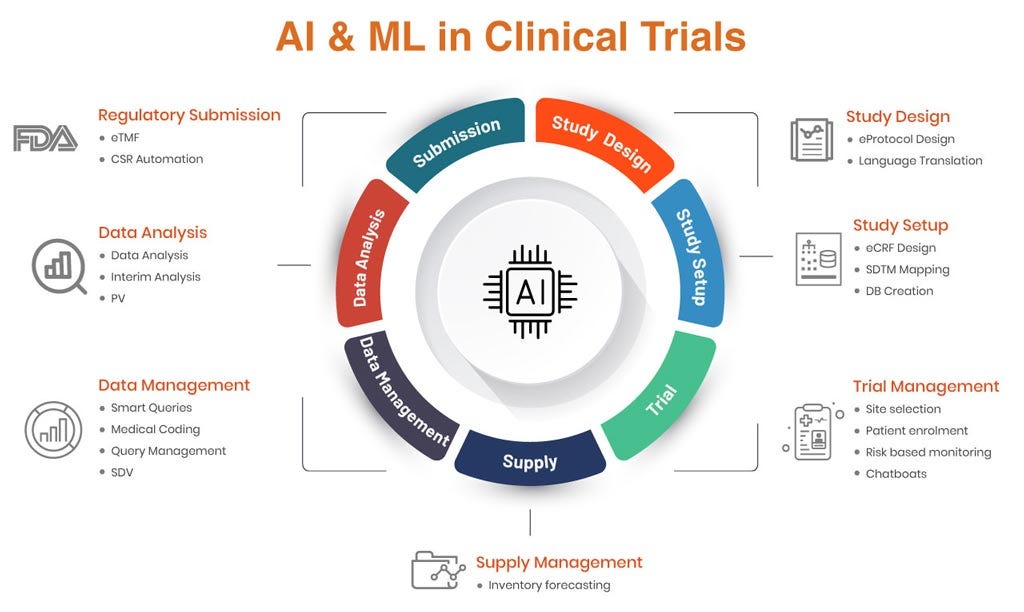
AI-Driven Data Management in Clinical Trials
Clinical Data Management (CDM) has always been the backbone of clinical research—ensuring accuracy, compliance, and integrity of trial data. But…
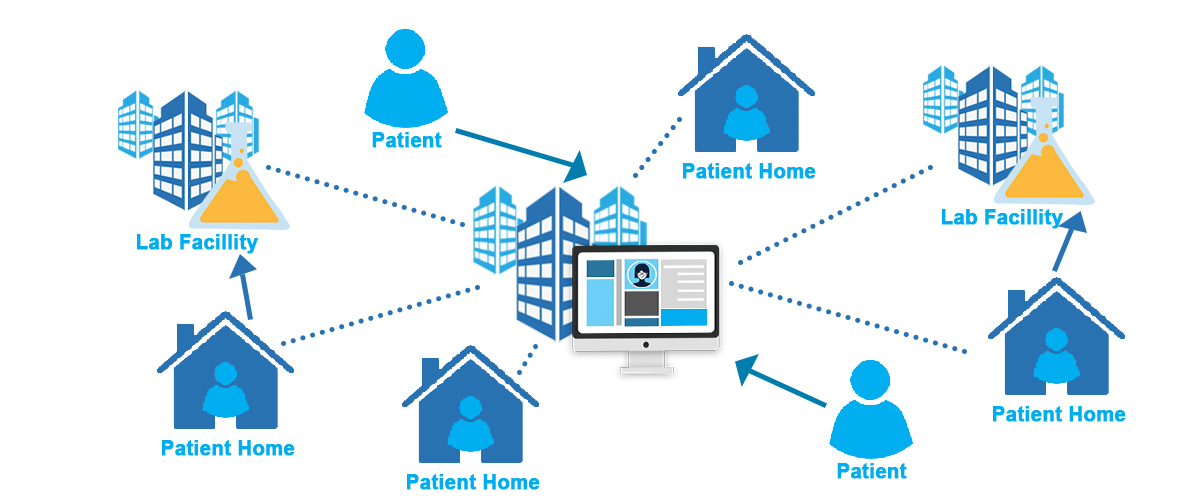
Decentralized & Hybrid Trials: Redefining Data Capture
The clinical research industry is undergoing a profound transformation. The move toward Decentralized Clinical Trials (DCTs) and hybrid models is…
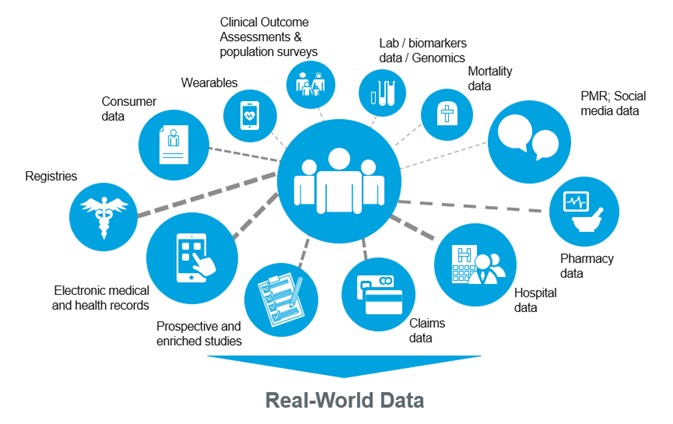
Real-World Data (RWD) & Real-World Evidence (RWE): Transforming Clinical Trials
In recent years, Real-World Data (RWD) and Real-World Evidence (RWE) have moved from being buzzwords to becoming central elements of…
Wearables & Patient-Generated Data: Managing Volume, Variety, and Value
The future of clinical research is being shaped by a single powerful trend: the rise of wearables and patient-generated health…
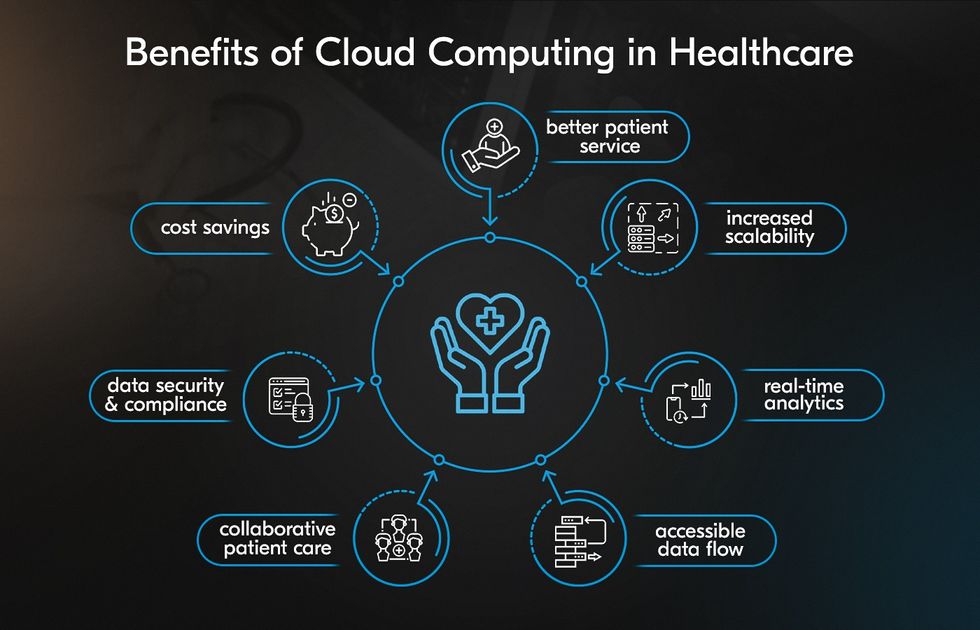
Cloud-Native & Scalable CDMS: Building the Data Foundations of Modern
The landscape of clinical trials is changing rapidly. With the rise of decentralized trials, wearable technologies, and real-world data integration,…
Pfizer Associate Safety Data Management Specialist Job 2025 in Chennai
Pfizer, one of the world’s leading pharmaceutical companies, is hiring for the role of Associate Safety Data Management Specialist in…



Merely a smiling visitant here to share the love (:, btw great layout.
I too think therefore, perfectly pent post! .