The future of clinical research is being shaped by a single powerful trend: the rise of wearables and patient-generated health data (PGHD). No longer limited to fitness enthusiasts, wearable technologies are now providing clinical-grade data that is transforming how trials are designed, monitored, and evaluated.
From smartwatches to advanced biosensors, the ability to continuously capture patient health data is enabling more patient-centric, decentralized, and efficient trials. But with opportunity comes the challenge of managing the volume, variety, and value of this data.
Real-World Impact on Patients
Wearables have changed the way patients participate in trials:
- Reduced site visits: Patients can stay at home while devices collect vital data such as heart rate, blood oxygen levels, or sleep patterns.
- Better safety monitoring: Real-time tracking enables faster detection of adverse events, allowing clinicians to intervene earlier.
- Higher engagement and retention: Patients who see their own data often feel more connected to the study, leading to improved compliance.
For example, in the Johnson & Johnson Heartline Study, the Apple Watch was used to detect atrial fibrillation in thousands of patients. This large-scale, virtual trial demonstrated both feasibility and the benefit of wearable-driven engagement.
Accelerating the Trial Process
Wearables do not just make trials more patient-friendly—they make them faster and more robust:
- Faster recruitment: Remote monitoring allows inclusion of patients from rural or underserved areas.
- Richer datasets: Continuous data capture provides a more accurate picture of patient health than occasional site visits.
- Adaptive designs: Real-time insights allow sponsors to adjust protocols or dosing strategies mid-trial.
For example, Pfizer leveraged Fitbit devices in neurological studies to monitor sleep and activity. This provided researchers with valuable, real-world insights that were not possible through traditional clinic visits alone.
Tools and Platforms Driving Adoption
Several leading companies are already embedding wearable data into their clinical data management workflows:
- Medidata Sensor Cloud: Offers a unified platform to ingest, normalize, and analyze data from multiple wearable devices.
- Veeva Vault CDMS: Provides cloud-native integration capabilities for external data streams, including wearable outputs.
- Clario: Specializes in clinical-grade sensor solutions widely used in respiratory and cardiovascular trials.
- Labcorp’s Covance: Supports decentralized trial models with wearable device integration for continuous remote monitoring.
These platforms are bridging the gap between consumer devices and regulatory-grade clinical datasets.
The Integration Challenge
Bringing wearable data into trial systems is complex. Challenges include:
- Volume of Data: Continuous monitoring can generate gigabytes of raw data per patient.
- Solution: Cloud-native CDMS platforms that can scale elastically to store and process high-volume datasets.
- Data Variety and Quality: Devices differ in format and accuracy, and patient misuse can create inconsistencies.
- Solution: Use AI-driven data cleaning and industry standards like CDISC ODM to normalize and validate inputs.
- Interoperability Issues: Proprietary device formats make it hard to unify data streams.
- Solution: Adoption of standards such as HL7 FHIR to enable seamless interoperability.
- Privacy and Compliance: Sensitive health data raises concerns under HIPAA, GDPR, and the European Health Data Space (EHDS).
- Solution: Strong encryption, audit trails, and patient consent frameworks built into CDMS platforms.
The Road Ahead
Wearables and patient-generated health data are no longer experimental—they are becoming a core component of modern clinical research. They benefit patients by reducing burdens, improve trial timelines through richer real-world evidence, and empower sponsors with actionable insights.
As cloud-native platforms, interoperability standards, and AI-based validation mature, the industry will be able to overcome today’s challenges and unlock the full potential of wearables in clinical trials.
The message is clear: wearable data is not just adding numbers to databases—it is adding real value to both patients and science.
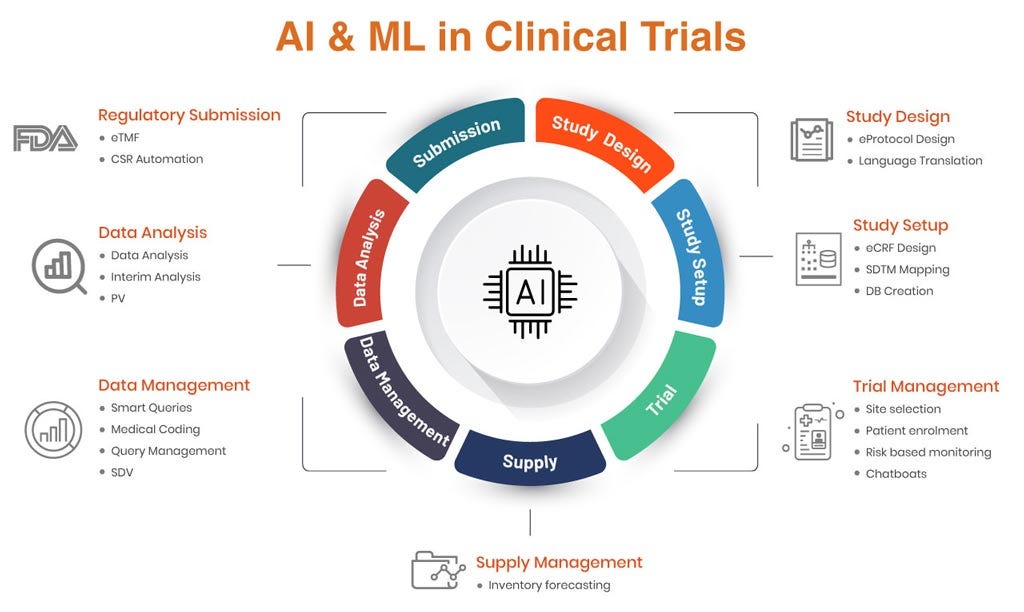
AI-Driven Data Management in Clinical Trials
Clinical Data Management (CDM) has always been the backbone of clinical research—ensuring accuracy, compliance, and integrity of trial data. But…
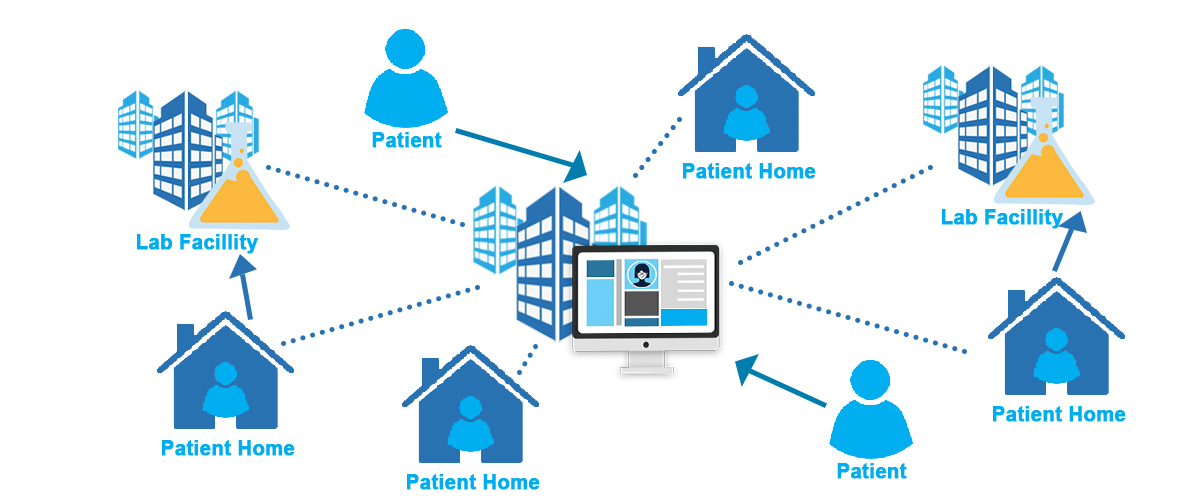
Decentralized & Hybrid Trials: Redefining Data Capture
The clinical research industry is undergoing a profound transformation. The move toward Decentralized Clinical Trials (DCTs) and hybrid models is…
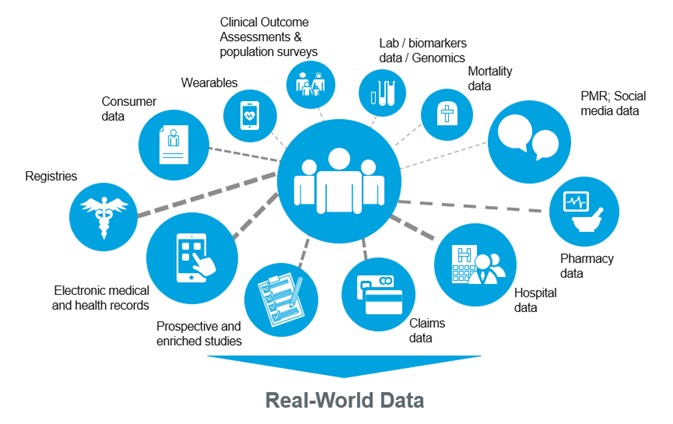
Real-World Data (RWD) & Real-World Evidence (RWE): Transforming Clinical Trials
In recent years, Real-World Data (RWD) and Real-World Evidence (RWE) have moved from being buzzwords to becoming central elements of…
Wearables & Patient-Generated Data: Managing Volume, Variety, and Value
The future of clinical research is being shaped by a single powerful trend: the rise of wearables and patient-generated health…
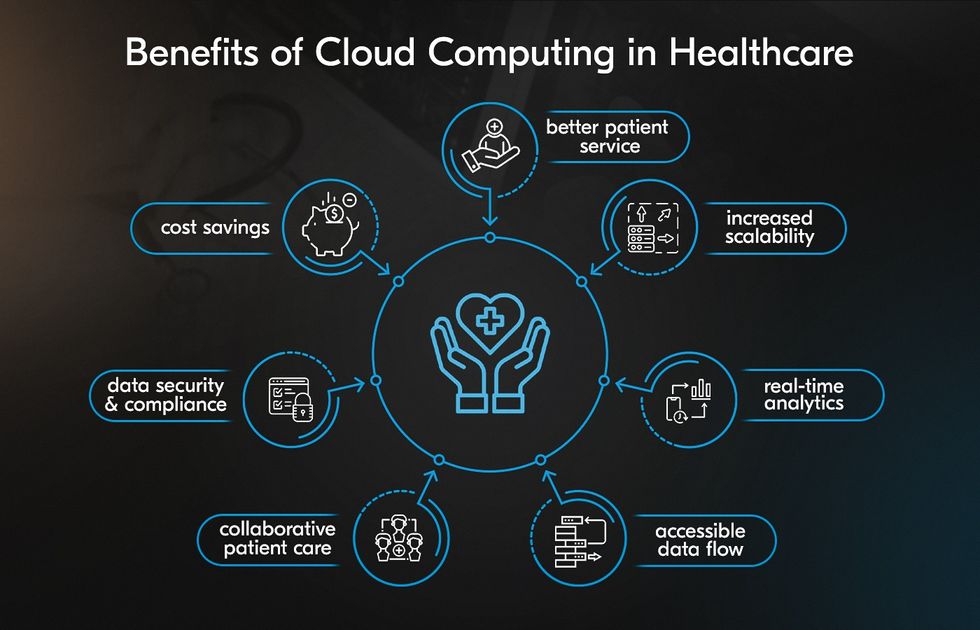
Cloud-Native & Scalable CDMS: Building the Data Foundations of Modern
The landscape of clinical trials is changing rapidly. With the rise of decentralized trials, wearable technologies, and real-world data integration,…
Pfizer Associate Safety Data Management Specialist Job 2025 in Chennai
Pfizer, one of the world’s leading pharmaceutical companies, is hiring for the role of Associate Safety Data Management Specialist in…



weed edibles no prescription required worldwide